Revolutionizing Technology With Graphene
By Veronica Villanueva, C2ST Intern, Rush University
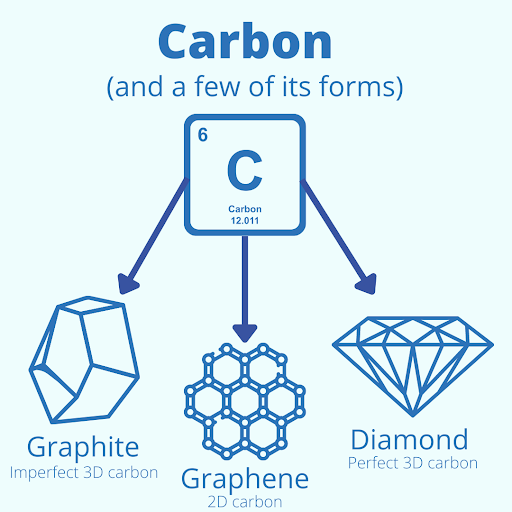
People will often call the end of a pencil the “lead tip,” but there is no lead in pencils. Pencils are filled with graphite, a crystalline form of carbon. Carbon is a fascinating element. It is found in many commonplace items like paper, food, and medicine. Even we are made of carbon. Carbon is essential. It creates extremely strong bonds and is tetravalent (it has 4 spare electrons to bind other elements) making carbon a perfect building block for our world.

Graphite forms when carbon binds to carbon. The atoms in graphite form an imperfect 3-dimensional hexagonal structure making it extremely stable, but when this same structure forms a perfect hexagonal structure under enormous pressure, the carbon becomes diamond. But pure carbon can take other forms, like graphene. Graphene is a 2-dimensional form of carbon where each atom binds to form a similar hexagonal structure often called a “honeycomb lattice.” Because of this 2-dimensional structure, carbon has almost limitless potential.
Graphene is stronger than steel despite being lightweight and transparent. Graphene can be used as a lightweight coating to increase the durability of objects and other materials. Graphene-coated tennis rackets and ski helmets are already available. Graphene is also exceptionally conductive, which means it has applications in electronics production. Engineers are developing graphene phone batteries which are expected to have 60% greater capacity compared to lithium ion batteries (meaning a fully charged phone could last for days). Graphene touchscreens would make electronics lighter and flexible and (potentially) cheaper to produce.
If graphene is so useful, why hasn’t it changed the world yet? The integration of graphene into technology–specifically consumer products–isn’t as seamless as we would hope. Graphene is a fairly new discovery–it was initially theorized to exist in the early 20th century, but it wasn’t fully identified until 2004. Meaning, there are still several steps to take before we put graphene in everyone’s pockets. Two major factors have affected the introduction of graphene into technology:
- There are several methods to produce graphene. These methods yield different levels of product quality. Good-quality graphene is necessary for use in phone screens and batteries. Engineers have to make enough graphene for everyone’s phones AND it has to be good quality. Not an easy task to accomplish.
- Graphene has no bandgap. Bandgap is the energy difference between a high energy state and a low energy state in electronics. It is necessary in semiconductive materials. Essentially, graphene is too conductive. Researchers are trying to artificially introduce bandgap into graphene, but that process needs to be scalable to be integrated into commercial products.
Overall, graphene has the potential to revolutionize technology. With the special characteristics of graphene, scientists believe it can be applied to solar energy and batteries, even medicine and water filtration. We just need to give engineers and scientists a few years to “iron out the wrinkles” so they can create the perfect 2D graphene we need. Patience is a virtue.
Sources
- https://en.wikipedia.org/wiki/Graphite
- https://www.graphene-info.com/graphene-introduction
- https://www.smh.com.au/sport/tennis/fasterstrongerbut-does-graphene-make-for-a-better-tennis-racquet-20150619-ghsod1.html
- https://www.androidauthority.com/graphene-batteries-explained-1070096/
- https://phys.org/news/2022-01-graphene-rare-metal-mobile-screens.html#:~:text=We%20have%20shown%20that%20a,indium%20is%20a%20sustainable%20material.
- https://www.science.org/doi/10.1126/science.1102896
- https://www.azonano.com/article.aspxArticleID=3677#:~:text=Reasons%20for%20Graphene’s%20Lack%20of%20Commercialization%20So%20Far&text=A%20bandgap%20is%20a%20range,applications%20of%20graphene%20are%20limited.